Bioink Review: Gelatin methacrylate, commonly referred to as gelMA, is a photocrosslinkable natural bioink derived from a hydrolytic degradation of collagen (1). GelMA is commonly used in drug delivery systems and wound dressing applications (1). With its cell encapsulation properties and thixotropic nature, gelMA is also a common reagent used for bioprinting (8). It has been used with a variety of cell types, including fibroblasts, chondrocytes (3), endothelial cells (4) and human umbilical vein endothelial cells (5).
Introduction
As a naturally derived reagent, gelMA has many properties useful in biological interactions, including its hydrophilicity, integrin-binding motifs, and matrix metalloprotein (MMP) degradation sites (5). Its highly hydrophilic nature allows it to dissolve in water or media, creating a hydrogel. With high water content, permeability to small molecules and high biocompatibility, hydrogels display good cell encapsulation qualities. Due to the integrin-binding sites, gelMA also displays high cellular adhesion. GelMA also has a pathway to degrade gradually within the body, as the MMP sites are degraded by enzymes, mainly certain metalloproteinases.
GelMA has a reversible thermal gelation and can be permanently crosslinked through photopolymerization. Before bioprinting, gelMA can be mixed with photoinitiators, such as LAP (2) or Irgacure 2959 (1,3). When exposed to a certain wavelength of light, the photoinitiators release free radicals that interact with the methacrylate groups on GelMA to create a solid gel through covalent bonds. Because of exposure to free radicals and sometimes harmful wavelengths, crosslinking time should be minimized to avoid effects on viability. This gelation process does not affect viscosity during extrusion and allows for tunable mechanical properties through post-process crosslinking, with an increasing elastic modulus with increasing crosslinking time.
In addition to crosslinking time, the mechanical properties of GelMA can be adjusted in a variety of ways. Varying the degrees of methacrylation during synthesis lead to varying compressive moduli, with increasing concentrations correlating to increasing compressive moduli (3). Additionally, increased gelMA concentration leads to increased elastic modulus and decreased mass swelling ratio (3). Ali Khademhosseini and his team of researchers tested gelMA concentrations ranging from 7-15%, establishing a link between printability and hydrogel properties (7).
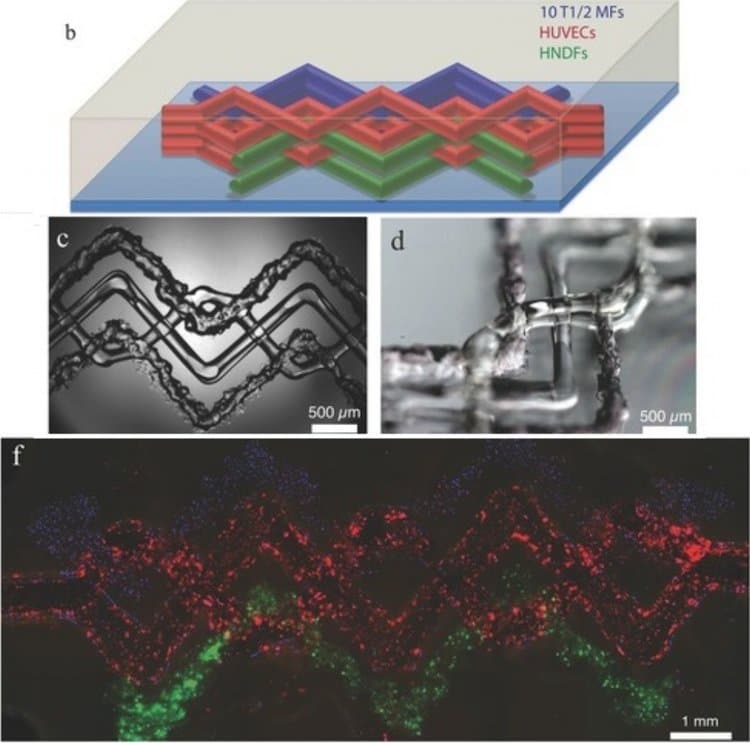
Researchers at Harvard University led by J. A. Lewis have also demonstrated the printability of gelMA through the fabrication of vascularized 3D tissue constructs. Lewis’s team, which printed gelMA with sacrificial pluronic F127, was successful in using their technique to create vasculature and maintain high viability up to 7 days after printing (5).
In addition to sacrificial reagents, GelMA has also been printed with hard thermoplastics such as polycaprolactone. Simultaneous printing with a thermoplastic, such as PCL or poly(propylene fumarate), increases the mechanical strength of a print, which is particularly beneficial for applications such as cartilage tissue engineering (3). This is known as a hybrid construct. For example, Dr. Z. Wang and his team of researchers used poly(propylene fumarate) to print and analyze the degradation of 3D scaffolds in bone tissue engineering applications (9).
GelMA has also been used in hybrid bioinks for enhanced properties. The addition of hyaluronic acid causes an increase in GelMA’s viscosity, allowing for improved properties for hydrogel printing (6). To increase mechanical strength, carbon nanotubes can be incorporated, as shown by Ali Khademhosseini and his team (10).
As a hydrogel, gelMA can be used as a very effective matrix bioink. It inherently has the beneficial properties of high cell adhesion and biocompatibility and tunable mechanical properties. This material offers further versatility when combined with a variety of components for specific purposes.
Materials and methods
In our shop we offer following GelMA related products:
Follow our dedicated protocols to prepare a bioink using a lyophilized or a sterile solution version of GelMA.
Before printing check out our detailed protocol for bioprinting GelMA.
Troubleshooting
My GelMA solution seems to have a low viscosity, or quickly dispenses out of my syringe.
Troubleshooting tip 1: Try placing your syringe on ice for 10-15 minutes before loading into the Allevi.
Prior to photocrosslinking, gelatin methacrylate has reversible thermal gelation, which causes the material to be liquid at 37 degrees celsius and a solid gel at room temperature (around 22 degrees celsius). Because of its shear-thinning properties, GelMA can be printed in this solid gel phase, offering some structural integrity prior to crosslinking.
If your GelMA appears to still be in liquid form when dispensing from the extruder (especially if it appears to gel a few minutes after extrusion), the loaded GelMA solution has likely not completely cooled to room temperature. Once loaded into the syringe, the solution can take a long time to cool to room temperature. Placing the syringe on ice prior to loading will help speed up this process.
Troubleshooting tip 2: Check your needle type, print temperature, and print pressure.
Check what needle type you are using and your print temperature and pressure settings. We suggest using a 27 gauge tapered metal Fisnar needle. Needles with a larger diameter will cause the material to extrude at a faster rate. Also, check your temperature and pressure settings. We suggest printing at room temperature (around 20-22 degrees celsius) and a pressure of around 10 psi.
When beginning a new print, it’s always best to calibrate your print pressure, first starting at around 0 or 1 psi and slowly working your way up to a pressure that causes an even extrusion. Print pressure can be affected by needle type, cell concentration and the volume of material loaded in a syringe, so it’s always best to start out with a lower pressure instead of accidentally dispensing most of your material with a higher pressure setting.
I have difficulty sterile-filtering my GelMA solution.
Troubleshooting tip 1: Make sure your GelMA solution is protected from light.
The first thing to check is that your GelMA has not prematurely crosslinked. Does your material still appear to be gelled even after heating it to 37 degrees celsius or higher? If so, it’s possible the solution, after being exposed to light, began to permanently crosslink. Try remaking your solution, being sure to protect it from any ambient light sources.
Troubleshooting tip 2: Try heating your GelMA to 60 degrees Celsius prior to filtration.
GelMA becomes slightly less viscous when heated to higher temperatures, making it easier to filter. Try heating it to 60 degrees celsius, as well as even warming your syringe and filter, prior to filtration, to make the process easier.
Troubleshooting tip 3: Try using a larger filter.
We suggest using a 0.2-micron syringe filter, but if you have significant trouble filtering the solution with this filter after trying the above steps, you can try using a larger 0.45-micron syringe filter, which will decrease the force needed to push the solution through the filter.
Troubleshooting tip 4: Try out our new sterile GelMA solution!
We know that preparing sterile GelMA is a headache! That’s why we offer pre-sterilized GelMA, available at our online store available in a ready-to-print solution or in lyophilized form!
My GelMA solution keeps clogging, or has an uneven extrusion rate.
Troubleshooting tip 1: Check what needle type you are using.
We suggest all-metal tapered 27 gauge tips (about a 0.33 mm diameter) when printing with GelMA. The all-metal tip helps prevent premature crosslinking in the needle before extrusion, which could cause clogging. Likewise, the tapered shape decreasing the pressure needed to print and helps to minimize clogging of the material.
Tips with a smaller diameter than 300 microns will quickly clog when using GelMA, while tips with a larger diameter will clog less. Even with these tips, however, GelMA can still occasionally clog the syringe. Readily available extra needle tips and some patience with the material are also suggested.
Troubleshooting tip 2: Add some hyaluronic acid to your solution.
Previous studies have demonstrated adding hyaluronic acid to gelatin-based formulations improves the consistency of extrusion and minimizes clogging. Depending on how much hyaluronic acid you add, you will lose some structural integrity (the ability to build in z-height without support materials), but this troubleshooting tip will help minimize clogging.
My GelMA isn’t crosslinking.
Troubleshooting tip 1: Make sure your gelMA solution or photoinitiator is not expired.
We perform quality control tests on every batch of gelMA that goes out the door, but the product can expire if kept at the wrong temperature or environmental conditions. You should use your gelMA within 6 months after receipt.
Additionally, after preparing the gelMA solution, you should use it within 24 hours.
Troubleshooting tip 2: Weak/defective photocrosslinker
Using a radiometer, check to see what intensity of light you’re receiving from your crosslinker. It should be from 5-10 mW/cm².
It’s also important to make sure that you are using the right wavelength for your photoinitiator.
References
- [1]Van Den Bulcke Al et al, “Structural and Rheological Properties of Methacrylamide Modified Gelatin Hydrogels,” Biomacromolecules, vol. 1, no 1, pp. 31-38, 2000.
- [2]Khademhosseini A et al, “Synthesis, properties, and biomedical application of gelatin methacryloyl (GelMA) hydrogels,” Biomaterials, vol. 73, pp. 254-271, 2015.
- [3]Malda J et al, “Gelatin-Methacrylamide Hydrogels as Potential Biomaterials for Fabrication of Tissue-Engineered Cartilage Constructs,” Macromolecular Bioscience, vol. 13, no. 5, pp. 551-561, 2013.
- [4]Khademhosseini A et al, “Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs,” Lab Chip, vol. 14, no. 13, pp. 2202-2011, 2014.
- [5]Lewis JA et al, “3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs,” Advanced Materials, vol. 26, no. 19, pp. 3124-3130, 2014.
- [6]Malda J et al, “25th Anniversary Article: Engineering Hydrogels for Biofabrication,” Advanced Materials, vol. 25, no. 36, pp. 5011-5028, 2013.
- [7]Khademhosseini A et al, “Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels,” Biofabrication, vol. 6, no. 2, 2014.
- [8]L. Djakovic, V. Sovilj and S. Milosevic, “Rheological Behaviour of Thixotropic Starch and Gelatin Gels,” Starch, vol. 42, no. 10, pp. 380-385, 1990.
- [9]Wang Z et al, “3D bioprinting for engineering complex tissues,” Biotechnology Advanced, vol. 34, no. 4, pp. 422-434, 2016.
- [10]Khademhosseini A et al, “Carbon Nanotube Reinforced Hybrid Microgels as Scaffold Materials for Cell Encapsulation,” ACS Nano, vol. 6, no. 1, pp. 362-372, 2012.